- Home
-
Products
-
Continence Care
-
Urology Care
-
Ostomy Care
- Stool Management System
-
Gynecology Care
-
Surgical and Respiratory
-
ViShield Epidemic Prevention & Control Solution
- Gloves
- Fingertip Pulse Oximeter
- Qxygen Concentrator 5L
- Qxygen Concentrator 10L
- Lotion Pump
- Plastic Trigger
- Disposable Face Mask VIFM01
- Disposable Medical Face Mask VIFM02
- KN95 face mask VIFM03
- Disposable Medical Coveralls
- Protective Goggles / Eye Shield VIES01
- Face Shield VIFS01
- Instant Hand Sanitizer
- Powder free disposable vinyl examination gloves
- Non-Contact Infrared Forehead Thermometer
-
COVID-19 VACCINE,ANTIGEN & Related
- COVID-19 ANTIGEN RAPID TEST CASSETTE
- CoronaVac Vaccine-SARS-CoV-2 (Vero Cell)Inactivated Vaccine
- SARS-CoV-2 Inactivated Vaccine (Vero Cell)
- ViShield Diagnostic kit for anti- 2019-nCoV IgM/IgG Antibodies
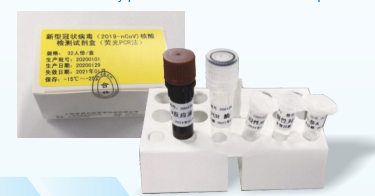
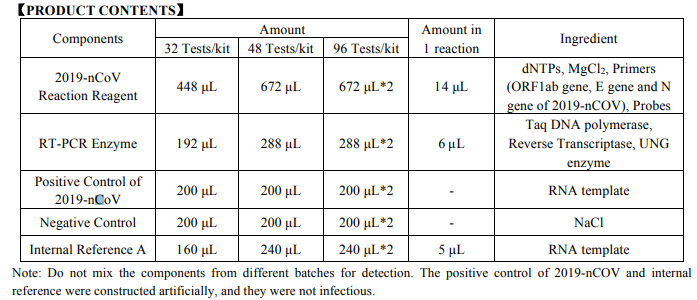
- 2019-Novel Coronavirus (2019-nCoV) RT-PCR Detection Kit
- AD Syringe for vaccine injection 0.5ml Syringe
- SAFETY BOXES 5.0L CARDBOARD SHARPS CONTAINER
-
Genetic Testing and Molecular Diagnostics
- Rotary Nucleic Acid Extractor-ViRotex 48
- Rotary Nucleic Acid Extractor-ViRotex 96
- Automated Nucleic Acid Extractor-Vitex
- Automated Nucleic Acid Workstation-VITAI 9600S
- Portable ATP Hygiene Monitoring System-Vitalum
- Automated Sample Processing System-VitaiMix 48
- Sample Collection Tube with Swab-T319
- Nucleic Acid Extraction Kit-T014H
- Real-Time PCR System-Vitier 96
- Real-Time PCR System-Vitier 48
- Real-Time PCR System-Vitesy 96T
- PCR Detection Kit-P742H
- Mobile PCR Lab-ViShield
-
Laparoscopic and endoscopic
-
ViShield DNA/RNA Collection Devices
-
ViSmart Medical Automation Solution
-
Biofoodpap PLA Food Pack
-
Continence Care
- Solutions
- About Us
- News
- Why Us
- Contact Us